Introduction
The Intestinal Flora Transplantation Clinical Research Foundation is a non-profit organization led by clinicians that works to establish a new treatment called “transplantation of intestinal bacteria (flora)” and contributes to human health through the clinical application and research and development of intestinal bacteria (flora) transplantation.
The transplantation team belonging to our research group has developed a unique method of transplanting intestinal bacteria (plexus) through more than 35 years of basic research on bacteria, and since its establishment in November 2017, our research group has performed more than 350 cases of intestinal bacteria (plexus) transplantation so far under the supervision of clinicians.
Although the number of transplants for autism spectrum disorder is still small (only 10 cases), reports of two particularly noteworthy cases among patients who have shown behavioral changes along with improvement in gastrointestinal symptoms, especially diarrhea and constipation, after transplantation were presented at the 4th Annual Meeting.
GASTROINTESTINAL SYMPTOMS AND GUT MICROBIOTA CHANGES INFLUENCED BY FMT IN AUTISM SPECTRUM DISORDERS
Autism is a neurodevelopmental disorder, now called Autism Spectrum Disorders (ASD). The main symptoms include difficulties with social behavior and interpersonal communication, repetitive behaviors, and a strong preoccupation with certain things.
The prevalence of autism has been estimated at 1-2%, but the number of patients is increasing not only in Japan but also worldwide, drawing attention and research as a social problem. The prevalence of autism has been estimated at 1 to 2%. .
In recent years, it has become widely known that the intestinal microbiota acts on the brain and has a variety of effects on the brain. Fecal microbiota transplantation (FMT), in which a healthy person’s balanced intestinal bacteria are transplanted into a person with a disease, is attracting attention as a method to improve digestive symptoms and social behavior in ASD.
About Various Inspections
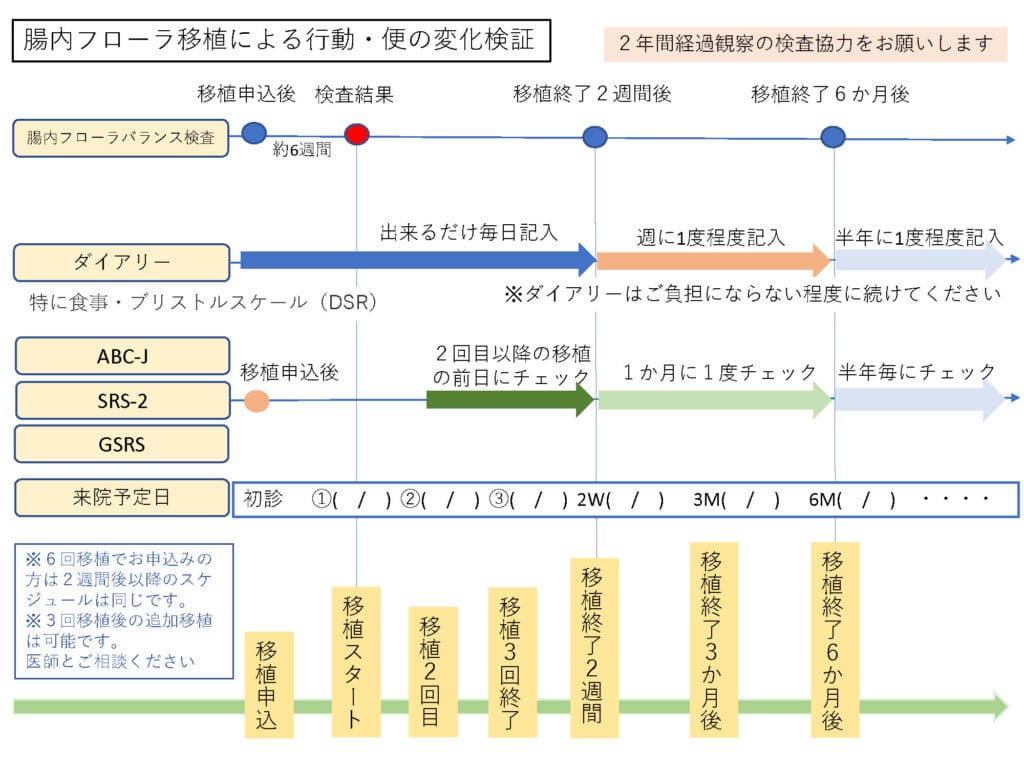
We have decided to request that parents/guardians of patients diagnosed with “Autism Spectrum Disorder” undergo the following tests when they receive a transplant.
ABC-J (Abnormal Behavior Checklist)
・SRS-2 (Interpersonal Responsiveness Scale Checklist)
・GSRS (Gastrointestinal Symptom Checklist)
・Bristol Stool Shape Scale (included in the diary)

Timing of various inspections
If you cooperate, we will waive the intestinal flora balance test (55,000 yen x 2 times).
(Please note that the intestinal flora balance test will only be free of charge in the verification of patients with autism spectrum disorder.
This trial is an opportunity to greatly expand the possibilities of intestinal bacteria (flora) transplantation by asking patients to record changes in behavior and stool conditions, and to verify from various perspectives what changes will appear with transplantation. This will be an opportunity to expand the possibilities of transplantation of intestinal bacteria (plexus). If you are willing to cooperate, please inform your doctor.
Partner Medical Institutions
(Kanto)
・Medical Corporation Futamatakai Natural Art Clinic ( Tokyo)
Director Dr. Yasuhito Mikawa
(Kansai)
・Medical Corporation Jinzenkai Tanaka Clinic ( Osaka)
Director Dr. Zen Tanaka
・Luke’s Ashiya Clinic (Hyogo)
Director Dr. Masahiko Johtani
・Medical Corporation Yuuakai Katai Internal Medicine Clinic ( Osaka)
Director Dr. Yuichi Kawai
・Medical Corporation Haruna Clinic ( Osaka)
Vice Director Dr. Reiko Haruna
(Chubu)
・Life Clinic Tateshina (Nagano)
Dr. Masayuki Aueholm, Director
(Tokai)
・Medical Corporation Keimeikai Kimura Hospital ( Aichi)
Director Dr. Mamoru Kimura
(Chugoku)
・Medical Corporation Iryushinkai Yorozu Clinic ( Tottori)
Director, Dr. Noriaki Man
(Kyushu)
・Medical Corporation Kiwakai Kitamura Clinic ( Fukuoka)
Director Dr. Kunihiro Kitamura
・Grace Medical Clinic ( Kumamoto)
Director Dr. Nobuhisa Ito
Provision of information and access to materials related to this research
If, during the course of your participation in this clinical application, we obtain new information that may affect your willingness to continue your participation in the study, we will notify you immediately. If we obtain important information regarding this research, we will again confirm your willingness to continue your participation in the research.
If you would like to see more detailed information about the research plan and methods, please consult your physician or other relevant personnel.
We will disclose such information to the extent that the personal information of other subjects is protected and the originality of this research is preserved.
Regarding third-party access to research records, etc.
In order to ensure that this research is being conducted properly while protecting the human rights of patients, the people involved in the research, government agencies, and other related parties may look at your medical records and other information. However, these parties are obliged to maintain confidentiality, so the patient’s name and other privacy-related information will be protected.
Handling of Personal Information
Your data obtained in this study may be published in medical journals, but your privacy will be protected because we will not reveal any personal information such as your name.
As for the information collected from the research data and medical records you have provided, we will exclude all personally identifiable information (name, date of birth, address, etc.) from these, and will instead attach a registry number for this study so that it is impossible to know who the stool sample and information belong to.
However, a correspondence table will be created and kept so that individuals can be identified when necessary, and the registration number can be linked to the individual. (This is called “linkable anonymization.”)
In this way, due attention will be paid to the protection of personal information.
Methods of storage and disposal of samples and information
Materials and data obtained in this study will be stored for at least 10 years from the final publication of the results of this study.
Careful consideration will be given to the protection of personal information when storing and disposing of data and other materials.
Requests during the transplant period
Please notify us if any of your medications change during the study period.
Please inform your doctor if you have taken any antibiotics or if a new intestinal regimen has been added or removed.

















