It is now widely known that the balance of microbiome is closely related to our health and the development of disease.
However, it is not easy to restore the microbiome balance that has been disrupted or to change it to the ideal state.
Therefore, there is growing hope that a treatment method called Fecal Microbiota Transplantation (FMT, fecal transplants, or gut flora transplants), that involves transplanting intestinal bacteria from healthy individuals can restore the balance of microbiome.
It is said by medical experts that “the gut microbiome is an ‘organ’. Given medical advances in restoring health by transplanting damaged organs, the transplant of gut microbiome is a natural step.
Unlike other organ transplants, commonly performed fecal microbiota transplantation are less painful, cause fewer side effects, are easier on both the donor and patient, and are widely useful in a completely different way than conventional treatment and prevention.
The target diseases for fecal microbiota transplantation are being verified around the world.
At present, published studies show that fecal microbiota transplantation have been performed for the following diseases.
- Recurrent Clostridioides difficile infection
- Ulcerative colitis
- Crohn's disease
- Irritable bowel syndrome
- Metabolic syndrome
- Autism spectrum disorder
In addition, diseases that have been linked to gut bacteria include:
- Atopic/allergy
- Cancer
- Type 1 and type 2 diabetes
- Dyslipidemia, obesity and hypertension
- Mental disorders
- Diseases related to pregnancy, childbirth and menstruation
- Other autoimmune diseases and lifestyle-related diseases
What is fecal microbiota transplantation?
The ecosystem of gut bacteria living mainly in the large and small intestines is officially called “gut microbiome”
The intestines are home to over 100 trillion gut bacteria of hundreds of species, and their composition is unique to each individual. The roles of gut bacteria are diverse, including immunity, mental stability, metabolism of the food we eat, tissue regeneration, and communication with organs throughout the body. Bacteria are responsible for minimizing damage to the body and mind and maintaining homeostasis by gradually changing the ratio of bacteria and the strength of their functions (balance) according to the state of our body and mind.
“All disease begins in the gut.”
Hippocrates, the “founder of medicine,” said. The balance of gut microbiome is essential for a healthy life and protects the important ecosystems of the body.
Advances in genetic analysis technology since 2000 have revealed that disruption of the gut flora balance is involved in various diseases. Due to westernization of food, overuse of antibiotics, excessive cleanliness, and stressful lifestyles, modern people are prone to gut flora imbalance. Not only constipation and diarrhea, which are easily imagined from the intestines, but also weight gain, allergic diseases, diabetes, renal diseases, and autism spectrum disorder are reported to be closely related to gut bacteria.
With a focus on the function of such gut microbiome, studies are being conducted worldwide on the effectiveness and safety of fecal microbiota transplantation in which gut bacteria collected from the stools of healthy people who meet certain criteria are administered into the colons of patients by means of an intravenous catheter, colonoscopy, duodenoscopy, or oral capsule.
What is the NanoGAS®-FMT method?
Purification of the transplant solution

The most unique feature of the NanoGAS®-FMT transplant solution is the use of NanoGAS® water for stool dissolution. NanoGAS® water has an overwhelmingly higher number of bubbles than ordinary nanobubbles, and its long-term stability and transportability can be utilized to produce a variety of effects.
By utilizing the characteristics of these nanobubbles, we have succeeded in effectively establishing the gut bacteria of other people’s origin, which were originally considered to be blocked by autoimmune functions such as IgA (immunoglobulin A), in patients.
7 NanoGAS®-FMT Features
1. Negative charge
Nanobubbles are negatively charged, so they repel each other and do not combine. They also attract and adsorb organic dirt that is positively charged. Since the bubbles are very small, they have a cross-linking effect that penetrates the underside of dirt and lifts it off the surface. This is also called the surfactant effect.
In gut microbiota transplants, this effect removes organic dirt from the intestinal wall and induces bacteria into the mucus layer of the intestinal tract without damaging the flagella and cilia of the transplanted gut bacteria.
2. Extended bacterial solution quality

The bubbles in NanoGAS water act as a buffer, inhibiting the exchange of information between bacteria and preventing overgrowth

Saline solution used for conventional FMT causes biofilm formation and the accumulation of dead bacteria.
Because the bubbles in NanoGAS® water are extremely small, they are not buoyant.
Therefore, the bubbles remain in the liquid for a long period of time without disappearing.
In gut microbiome transplants, NanoGAS® water exhibits preservation performance equivalent to freezing. This prevents bacteria intaracting and distorting the state of the bacterial fluid into an unintended balance before transplants.
3. Donor selection

Fecal microbiota transplantation currently undergoing clinical trials in Japan often require finding a healthy donor within a second degree of kinship to alleviate psychological resistance to the transplant of bacterial solution purified from stool. On the other hand, in the United States and United Kingdom, stool banks have been established and FMTs are performed using stools from healthy unrelated third parties.
We believe the balance of diverse gut microbiome is important and FMTs are more effective when using third-party donors with different genetics and living environments. We therefore use stool samples from Japanbiome, a donor bank affiliated with Shinbiosis, for the NanoGAS®-FMT procedure. Japanbiome only registers donors who have passed regular lifestyle management interviews, strict medical interviews, and blood and stool tests.
Donor bank Japanbiome is affiliated with Shinbiosis Inc.
4. Transplantation method
Number and frequency of transplants
Currently, only patients with CDI (Clostridioides difficile infection) are officially approved by the U.S. government to receive fecal microbiota transplantation. With CDI, clinical trials are basically conducted with only one transplant.
Other diseases such as ulcerative colitis and autism spectrum disorder have different protocols and clinical trials are being conducted.
With NanoGAS®-FMT, we believe patient age and years of disease are more closely related to the outcome than the number of transplants. The attending physician and patient decide whether additional transplants are necessary after three to six transplants.

5. How to store stools
The NanoGAS®-FMT method does not use fresh stools that have not been tested and whose safety has not been assured. Only frozen and preserved stools are used after the health of the donor and the safety of the feces have been sufficiently confirmed. In addition to safety concerns, there is a concern that the method of using fresh stools would not deliver transplants to a large number of people.
Some reports have shown no significant difference between the results of FMT performed with fresh and frozen stools. We place the highest priority on safety and use frozen preserved stools. Since the effects of additives on the human body and bacteria are unknown, we do not add cryoprotectant (glycerol), which is commonly used as an enema solution.
Concentration of stool
The concentration of bacteria solution in foreign treatment is generally 1:5 and diluted with stock solution and saline solution, but this concentration may be too high.
In NanoGAS®-FMT, the bacterial solution is between 1:45 and 1:10.
To reduce the phenomenon of temporary confusion when the patient’s immune system recognizes the difference between the intestinal bacteria of others and the patient’s own intestinal bacteria, multiple transplants are performed in a short period of time in a systematic manner. Another feature of this method is to gradually increase the concentration of bacteria to ensure effective retention.
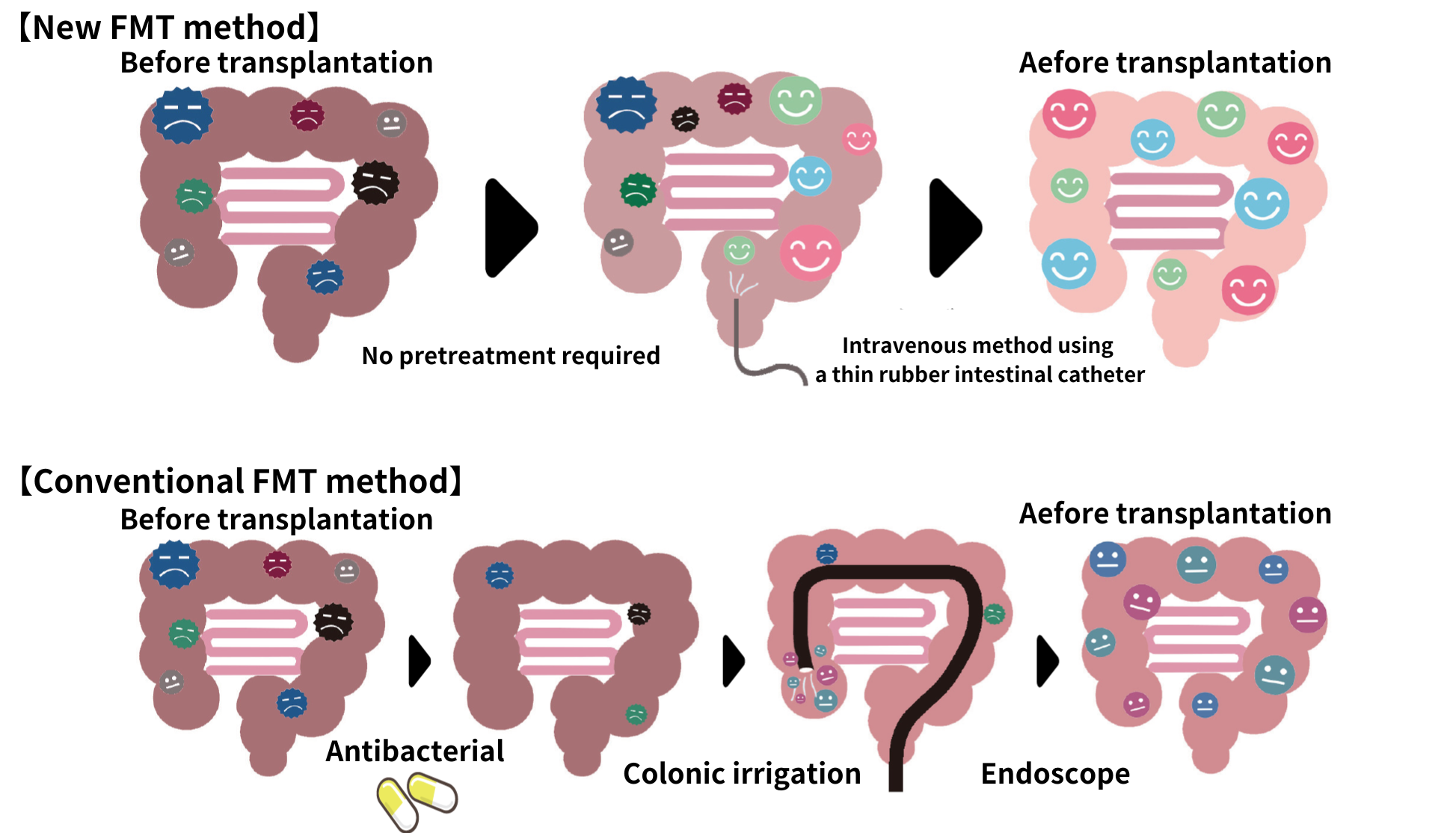
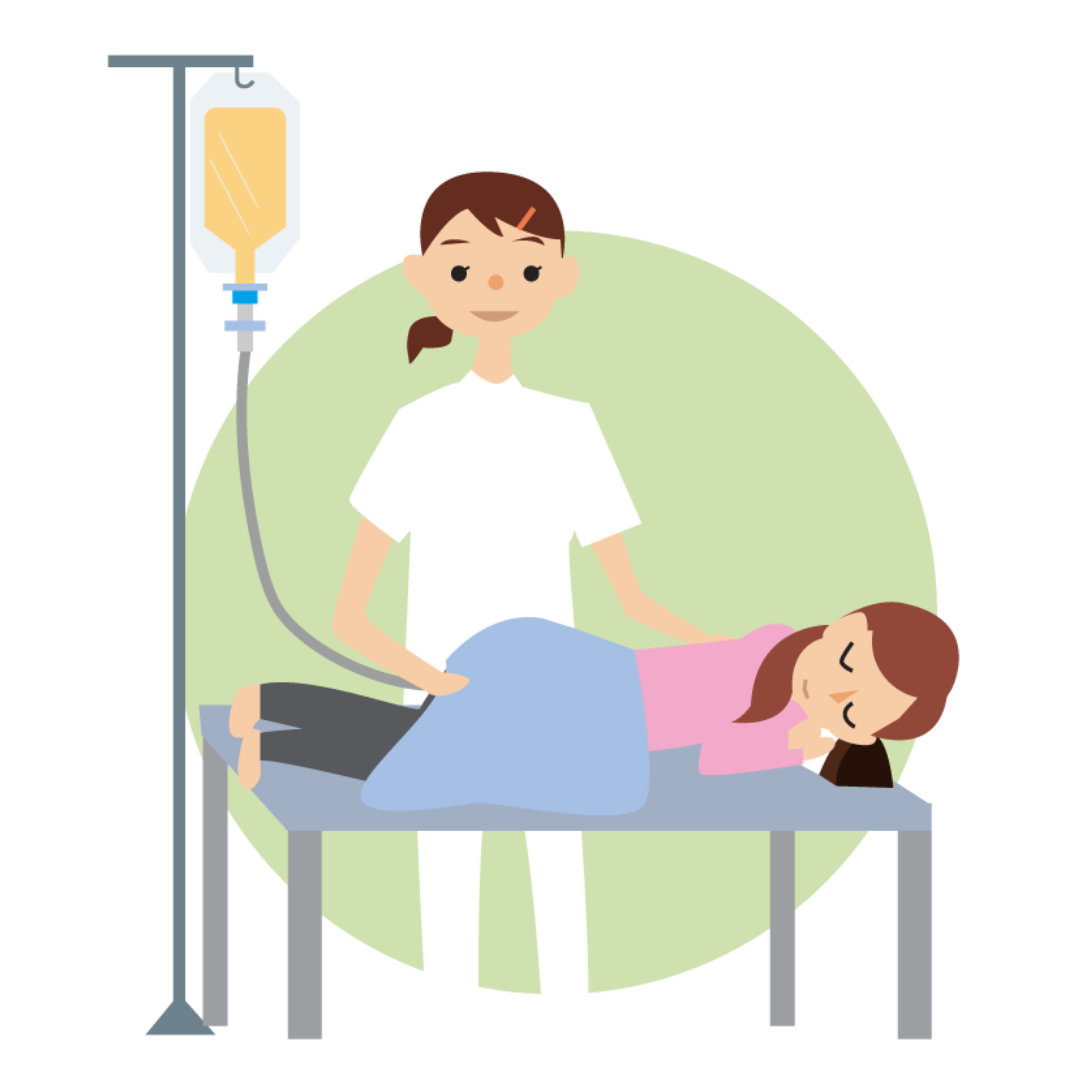
6. Administration method
Conventional methods include oral capsules and colonoscopy. However, oral capsules are large and require the use of stomach acid suppressants to prevent bacteria loss in stomach acid. In addition, colonoscopies require dietary restrictions and laxatives, both of which involve physical and mental pain.
The NanoGAS®-FMT procedure uses a soft rubber catheter implant to fully deliver the bacteria to the intestinal tract with little physical or emotional distress to the patient.
7. Pre-treatment or no pre-treatment
Many other clinical trials and clinical applications involve the use of gastric acid suppressants and bowel cleansing agents, as well as dietary restrictions prior to transplantation. The most common method is to take antibiotics for a certain period of time to impair the diversity of gut microbiome before a transplant.
In the NanoGAS®-FMT procedure, there is no such pre-treatment.
A list of clinics in Japan that have the knowledge, optimal screening, and superior medical technology to treat the disease can be found here.
Fecal transplants overseas
Establishment of the Fecal Microbiota Transplantation National Registry
U.S. initiatives
OpenBiome, the largest stool bank in the U.S., provides safe donor stool to medical institutions in the country (limited to CDI as the target disease). According to its website, it has provided more than 62,000 donor stools to date. A national guideline steering committee convened in 2017 to work with various research institutions and academic societies to compile guidelines for fecal microbiota transplantation.By February 2024, we will have provided more than 70,000 FMT bacteriological solutions, saving the lives of many CDI patients. We are also conducting clinical trials for more than 30 different diseases and have a substantial number of publications.
Australian Initiatives
In Australia, there are already 12,000 cases of FMT as an advanced medical treatment in 2020. 2021 onwards, FMT will be treated as a biopharmaceutical and the laws and regulations are changing. In 2022, Biomictra, the world’s first FMT-approved drug for BiomeBank, was developed and is currently being offered to patients with recurrent Clostridioides difficile infection (rCDI). BiomeBank has established a technology to create artificial stools that do not rely on donor stools and aims to transition from donor donations to artificially cultured microorganisms in the future.
Translated with DeepL.com (free version)
European initiatives
In Europe, a European Consensus Conference on Enterobacteriaceae Transplantation in Clinical Practice was held in 2017 by 28 experts from 10 countries. FMT stool banks are being developed in various countries and are mainly operated by university hospitals. The NDFB in the Netherlands and the MTC in the UK, for example, have established international FMT standard methods and are contributing to the treatment of Clostridioides difficile infection (CDI). On the other hand, official information is limited in countries such as Germany and Spain. Overall, each country is pursuing its own initiatives based on the guidelines, although a unified management system like that of the U.S. and Australia is not yet in place.
The usefulness of fecal microbiota transplantation and their history in Japan and abroad
Fecal transplants are currently being studied at various institutions in Japan and abroad, and a certain level of efficacy has been recognized in the treatment of ulcerative colitis and irritable bowel syndrome. However, in Japan the transplants are not yet covered by the Clinical Research Act. So the final evaluation has not yet been made and the patient must pay the full amount of the transplant.
The following is a summary of the history of the usefulness of fecal transplants in Japan and abroad to date. New information will be posted on this site’s blog as it becomes available.
The earliest fecal transplant is said to have been performed around the 4th century in China when a person suffering from diarrhea that would not stop was cured by placing stool from a healthy person in his buttocks. Since then, research on fecal microbiota transplantation has progressed in Western countries, starting with a report on pseudomembranous enteritis in 1958.
Fecal microbiota transplantation reentered the spotlight in 2013 when a Dutch study showed that it was effective in treating Clostridioides difficile infection (CDI), a disease caused by the use of large amounts of antibiotics in surgical procedures. This disease causes a loss of diversity in the gut microbiome, which should normally contain thousands of species, and in the worst case scenario the disease can be fatal. In the United States, more than 500,000 people are affected by this disease every year, and 30,000 people die each year.
The following year, in 2014, the U.S. Food and Drug Administration (FDA) positioned “fecal transplants as the treatment of first choice in cases of multidrug resistance in CDI.” Its effectiveness is currently being medically proven.
On the other hand, in 2019, the FDA issued a warning that “fecal transplants may pose a serious risk of infection” in cases where lethal bacteria were transplanted without proper screening at the time of transplantation and may result in death.
As of 2021, fecal microbiota transplantation exist under the FDA’s executive discretion policy and can be used to treat CDI. However, it is difficult to obtain the benefits of this new treatment without the provision of a reliable bacterial solution and the selection of a specialized hospital that can provide accountability.
Research on fecal microbiota transplantation in Japan began in 2013 at eight university hospitals and other facilities around the country, with clinical trials focusing on ulcerative colitis and Crohn’s disease (the first phase was completed in 2016). The effects on inflammatory bowel disease are being reported in turn.
A list of clinics in Japan that have the knowledge, optimal screening, and superior medical technology to treat the disease can be found here.
TRENDS IN CLINICAL TRIALS AND CLINICAL STUDIES OF FMT (JAPAN)
National Institute of Health Sciences, Clinical Research Information Portal Site, all 28, 2022.09.09)
| Disease | Number of cases | Research institution |
|---|---|---|
| Ulcerative Colitis | 9 | Juntendo University, Fujita Health University, Keio University, Shiga Medical University |
| rCDI | 7 | Shiga University of Medical Science, Osaka City University, Fujita Health University, Nagoya University |
| Crohn’s disease | 4 | Fujita Health University, Shiga University of Medical Science, Keio University |
| After allogeneic hematopoietic stem cell transplant | 3 | Shiga University of Medical Science (Cancer, Infectious Disease Center), Komagome Metropolitan Hospital |
| Immune Checkpoint Inhibitor Therapy |
9 | Fujita Health University |
| Other | 4 | Kanazawa University |
*2014-2021
*Completed: 5 UC, 1 rCDI
*Discontinued : 3 rCDI clinical studies
*Number of cases: 10, 20, 30, 50, 60, less than 30
The NanoGAS®-FMT method reports all adverse events.
Adverse Event Reporting
Safety system details:
Japanbiome affiliated with Symbiosis Inc.














