We are no longer accepting applications for children with autism spectrum disorder between the ages of 5 and 12 years old to participate in our clinical research. Thank you very much for all the inquiries.
The interim report of this clinical research will be presented at the 7th Intestinal Flora Transplantation Clinical Research Meeting on September 17. We would like to ask for your continued understanding and support in order to establish this as a medical treatment that anyone can receive with peace of mind.
Click here for information on the conference
https://fmt.sym-biosis.co.jp/news/event/9493/

1. Introduction
Clinical research refers to studies in which patients participate and cooperate to investigate the safety and efficacy of therapeutic and diagnostic methods. Many of the treatments and diagnostic methods currently in use have been advanced through clinical research in Japan and abroad.
Unlike “clinical trials,” which are conducted by companies, this research is conducted by researchers (physicians).
This clinical research is to be conducted after approval by an accredited clinical research review committee, approval by the director of each medical institution conducting the clinical research to conduct the research, and submission of the implementation plan by the principal investigator of the clinical research to the Minister of Health, Labor and Welfare.
2. autistic spectrum disorder
AUTISM SPECTRUM DISORDER (ASD) IS A DEVELOPMENTAL DISORDER CHARACTERIZED BY DIFFICULTY WITH INTERPERSONAL RELATIONSHIPS, OBSESSIVENESS, AND SENSORY OVERSENSITIVITY.
ASD is the name of a disorder that was announced in 2013 in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), the fifth edition of the American Psychiatric Association’s diagnostic criteria. Since then, ASD has come to be used as a collective term for disorders that had previously been referred to by various names, such as autism, pervasive developmental disorder, and Asperger’s syndrome.
ASD is said to be a brain dysfunction caused by a complex combination of factors.
The number of cases of ASD is increasing every year in Japan and abroad, and it has become a major social problem.
THE CAUSES OF ASD HAVE NOT YET BEEN IDENTIFIED, AND NO FUNDAMENTAL TREATMENT HAS BEEN DEVELOPED, BUT THERE ARE SEVERAL METHODS TO ALLEVIATE SYMPTOMS AND PROBLEMS CAUSED BY ASD, SUCH AS “ENVIRONMENTAL ADJUSTMENTS TAILORED TO ASD SYMPTOMS” AND “COUNSELING/PSYCHOTHERAPY. HOWEVER, THESE METHODS HAVE NO IMMEDIATE EFFECT, AND THERE ARE FEW SPECIALISTS WHO CAN IMPLEMENT THEM.
Medications include atypical antipsychotics (e.g., risperidone, aripiprazole), mood stabilizers (e.g., valproic acid), and psychostimulants, but these are effective only for the marginal symptoms of ASD and have not been shown to be effective for the core symptoms.
Thus, there is a strong need for the development of new treatments with faster onset and greater efficacy.
What is intestinal microflora transplantation?
FECAL MICROBIOTA TRANSPLANTATION (FMT) IS ALSO KNOWN AS FECAL MICROBIOTA TRANSPLANTATION OR INTESTINAL FLORA TRANSPLANTATION. IT IS A TREATMENT IN WHICH A FECAL MICROBIOTA SOLUTION PREPARED FROM HEALTHY HUMAN FECES IS TRANSPLANTED INTO THE PATIENT’S INTESTINE WITH THE AIM OF IMPROVING THE INTESTINAL MICROBIOTA.
The new FMT method (hereafter referred to as the NanoGAS-FMT method) does not require antimicrobial administration or bowel cleansing prior to FMT, which is commonly done with conventional methods. Instead of the saline solution used in the conventional method for dissolving stools, stable hydrogen nanobubble water, originally developed by Symbiosis, is used,This is a less burdensome method for children that does not use an endoscope and uses a thin, rubber-like tube to inject the rectum.
It is also being developed as a new method that uses a small dose, “about 1/1000th of the dose (number of bacteria) used in the conventional method.
Figure 1: Differences between the NanoGAS-FMT method and conventional FMT methods (conceptual diagram)
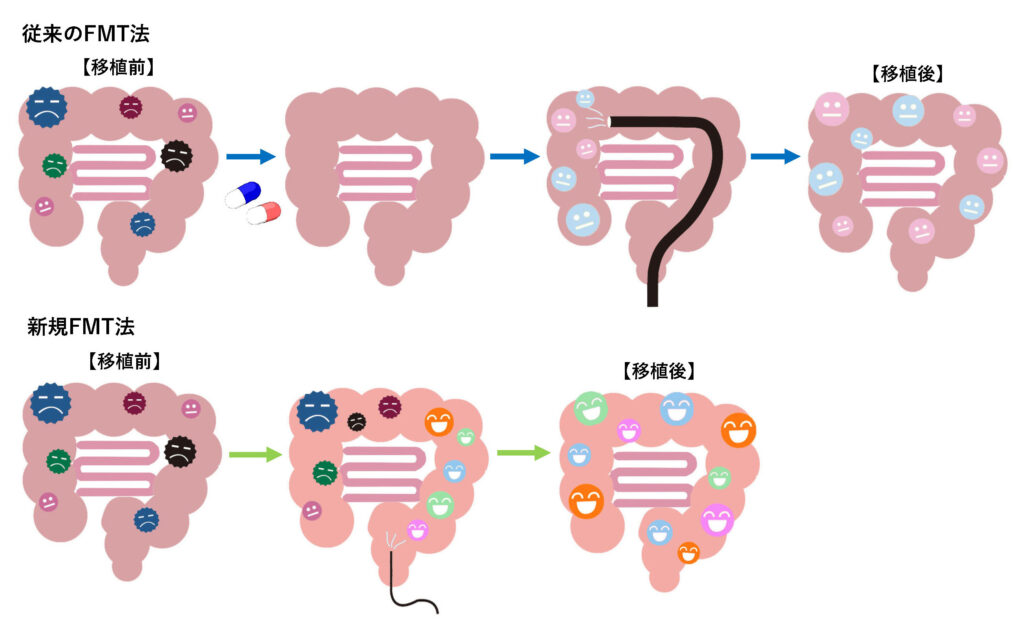
The differences are as follows
(1) NanoGAS-FMT method
* A THIN, SOFT, RUBBER-LIKE TUBE IS USED TO ADMINISTER THE FECAL MICROBIOTA SOLUTION INTO THE RECTAL AREA NEAR THE ANUS OF THE ASD CHILD. 3-5 MINUTES IS ALL IT TAKES. THE TRANSPLANTATION IS NOW COMPLETE.
(2) CONVENTIONAL FMT METHOD (EXAMPLE FROM UNIVERSITY OF ARIZONA)
- * Antimicrobial/vancomycin was administered daily for 2 consecutive weeks.
- * TWO WEEKS LATER, THE BOWEL IS CLEANED WITH INTESTINAL CLEANSER PEG.
- * A large amount of fecal microbial solution (more than 1000 times the amount for the NanoGAS-FMT method as the number of bacteria) is administered orally (or rectally) daily for 2 days.
- * A capsule formulation containing approximately 10 times the initial dose of the NanoGAS-FMT method as the number of bacteria was administered continuously for approximately 60 days.
- * At the same time, gastric acid secretion inhibitors were administered continuously for approximately 60 days.
In November 2022, the U.S. Food and Drug Administration (FDA) approved Rebyota, a fecal microbicide developed by Ferring Pharmaceuticals, for the treatment of recurrent Clostridioides difficile infection. This is expected to accelerate clinical studies and trials of FMT for various diseases in the future.
https://www.ferring.co.jp/category/global-2022/
IN THE PREVIOUS STUDIES CONDUCTED IN THE U.S. AND CHINA ON “FMT FOR THE TREATMENT OF CHILDREN WITH ASD,” AND IN THE ONGOING “CLINICAL TRIALS ONE STEP FURTHER FROM CLINICAL RESEARCH” IN THE U.S. AND CHINA, THE AGE OF STUDY SUBJECTS WAS REDUCED TO 2 AND 3 YEARS OLD, RESPECTIVELY, AS SHOWN IN THE TABLE BELOW.
IN THE CASE OF THE UNITED STATES, THESE ARE APPROVED BY THE FDA. SOME ARE ALSO APPROVED BY THE CFDA IN THE CASE OF CHINA, WHICH IS CONSIDERED THE MOST STRINGENT.
Table Age Range of Study Subjects in Prior Studies and Ongoing Clinical Trials
| implementing agency | stage | Age Range of Study Subjects |
| Univ. of Arizona | Prior Clinical Studies | 7-17 years old |
| Third Military Medical University | Prior Clinical Studies | 3-17 years old |
| ProgenaBiome | clinical trial | 2 years old and up |
| Ventura C.Trials | clinical trial | 2 years old and up |
| LA CHILDREN’S HOSPITAL | clinical trial | 5-17 years old |
| Univ. of Arizona | clinical trial | 5-17 years old |
| Wuhan University | clinical trial | 3-18 years old |
We have already received approval from the Ethics Review Committee and have initially applied the above “NanoGAS-FMT method” to the treatment of 14 children with ASD aged 5 to 12 years old.
After obtaining consent from the child’s parents, we analyzed the results of this free treatment and found that the NanoGAS-FMT method reduced the child’s ASD symptoms.
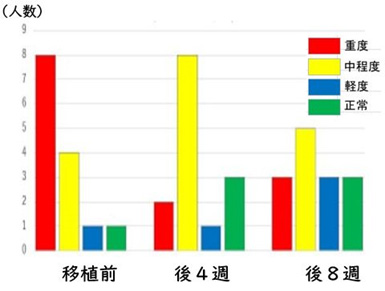
FIGURE 2 SHOWS THE CHANGE IN SEVERITY BY SRS-2 WITH RESPECT TO INDIVIDUAL CASES.
The NanoGAS-FMT procedure significantly reduced ASD symptoms in 10 of the 14 patients (71%), including 6 patients with severe symptoms, at 8 weeks post-transplant.
FIGURE 2: EFFICACY OF “FMT METHOD WITH SHIN-1” FOR ASD
As a result, in the above treatment results using 14 patients, adverse events of fatigue and constipation were observed in one patient, but these were minor and temporary.
4. research methods
(1) Eligible children
This study is open to children diagnosed with ASD who meet the following criteria.
Please note that you may not be able to participate depending on the judgment of your physician.
【Conditions for children who can participate in the program】
・ Children between the ages of 5 and 12 years old who have been diagnosed with ASD by a pediatric neurologist, a board-certified neuropsychiatrist, a board-certified child and adolescent psychiatrist, or a board-certified child psychiatrist.
(Medical certificate required*1; gender is not required)
・Children who are judged by the principal investigator to be in generally good physical health, except for gastrointestinal symptoms
・Children who are judged by the principal investigator to be in generally good physical health, except for gastrointestinal symptoms
・Agreed consent to participate in this study in writing can be obtained from the surrogateEnd
*1: If you have not yet obtained a medical certificate, please check this site to obtain one.
List of pediatric neurologists certified by the Japanese Society of Child Neurology:
https://www.childneuro.jp/modules/senmoni/
List of specialists certified by the Japanese Society of Psychiatry and Neurology:
https://www.jspn.or.jp/modules/senmoni/
List of specialists certified by the Japanese Society of Child Adolescent List of board-certified psychiatrists by the Japanese Society of Child and Adolescent Psychiatry:
https://child-adolesc.jp/nintei/ninteii-ichiran/
List of board-certified pediatric neuropsychiatrists by the Japanese Society of Child and Adolescent Psychiatry:
https://www.jsppn.jp/physician
[Conditions for children who are not allowed to participate
・Those who are currently undergoing drug treatment.
Patients who have received antibiotics (except topical antibiotics) within the past 3 months
・Patients who have had FMT within the past 12 months
・Single gene disorder with ASD-like symptoms (Fragile X syndrome, Rett syndrome, Joubert syndrome, or other conditions that may affect efficacy determination) Diseases that may affect the efficacy determination).
・Individuals with neurosurgical or neurological diseases.
・Individuals on tube feeding.
・Individuals who are underweight/undernourished below 65% of standard body weight.
・Individuals who have had surgery within one year or are scheduled to have surgery within six months.
・Currently participating in another clinical trial.
・For other reasons, Those who have been determined by their physician to be inappropriate to participate in the study.
(2) Study design
We will evaluate the efficacy and safety of the “NanoGAS-FMT method” by comparing test values before and after administration of the intravenous solution. For ethical reasons, this will be done without a subject group.
(3) PROCEDURE OF INTESTINAL MICROFLORA TRANSPLANTATION (FMT METHOD)
(1) Donor health, donor stool safety, storage and use of donor stool
(Donor: A person who donates fecal matter)
(1) Donor Health Status
The stool to be used is provided by a registered volunteer donor. The physician in charge of the donor will check the donor’s health status based on a medical examination and tests (a questionnaire with approximately 150 items and the following extensive blood tests) conducted once every two to three months.
<Medical Questionnaire>
Age, gender, height, weight, history of antibiotic use, natural childbirth/breastfeeding, medical history, drug or supplement use, travel history, eating and sleeping habits, etc.
<Doctor’s examination>
* Blood pressure, pulse, auscultation, urinalysis
* General blood tests:
* Biochemistry and immunology tests:
* Infectious disease tests:
* Immune function tests:
* Tumor marker tests:
(2) Donor stool safety
Stool tests are also conducted for bacterial resistance, food poisoning, and immunology, as well as for each of the fine print of genetic testing for bacteria to confirm safety.
*Genetic analysis of 16S rRNA intestinal microflora
*Bacterial resistance testing
*Food poisoning testing
*Immunological testing
(3) Storage and use of donor stools
Stools deemed safe are stored at -80°C. The frozen stools are used at the next inspection, which is performed once every 2-3 months as described above, when they are deemed acceptable by the physician.
This undiluted preparation process is performed at Symbiosis under investigational new drug GMP, an environment similar to that used in the manufacture of pharmaceuticals.
All operations are performed in a safety cabinet that is completely free of contamination.
Safety cabinet for preparing undiluted solutions

(2) Preparation and dosage of intravenous solution
The following preparation operations are performed at the medical institution
Since some children may be resistant to the administration of intravenous solution, we used a method of administration in which the concentration of the intravenous solution is thin at first, and then gradually increases, so that the child can become accustomed to intravenous administration.
(3) Method of administering intravenous solutions
The intravenous solution is administered intravenously once a week for six consecutive weeks, for a total of six intravenous administrations, each lasting three to five minutes. The total time required for one visit, including other examinations, is 30-45 minutes.
A thin rubber tube (6.5 mm in diameter) is also used during intubation. This is a less invasive method, although it may be uncomfortable for children with sensory sensitivity.
A final evaluation of efficacy is made with results at 30 weeks after the start of administration.

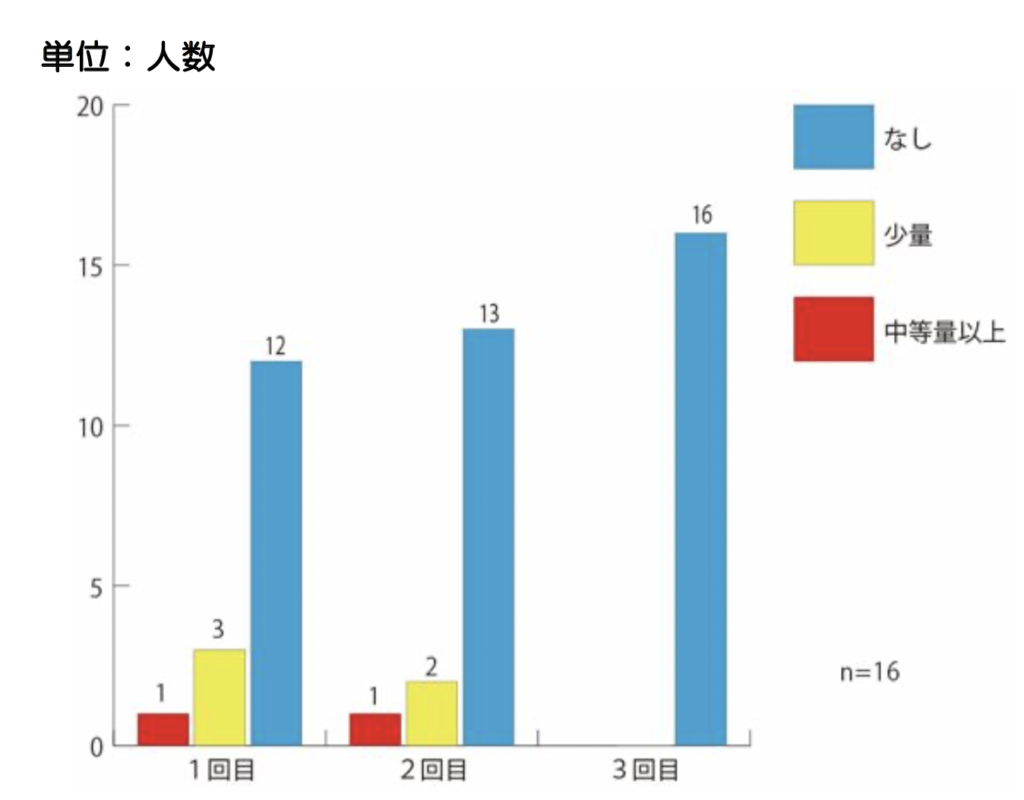
WE HAVE BEEN ADMINISTERING A FECAL MICROBIOTA SOLUTION (SHIN-1) INTRAVENOUSLY TO 16 INFANTS. THE DEGREE OF RESISTANCE IS INDICATED BY THE DEGREE OF CRYING (FIGURE 5) AND BY THE LEAKAGE OF SOLUTION DUE TO RESISTANCE (FIGURE 6). THE RESISTANCE SEEMS TO DECREASE WITH EACH ADMINISTRATION.
Figure 4: Degree of resistance (crying) during intubation
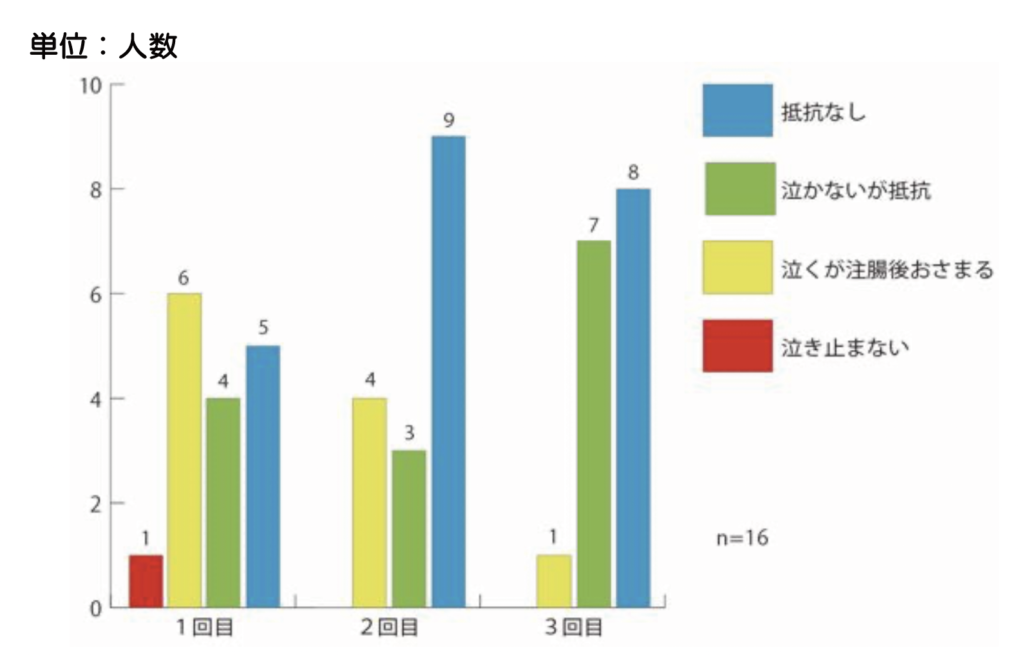
Figure 5: Degree of resistance during pouring (leakage due to violence)
(4) Inspections
(1) Tests to be performed at home
The following four tests are all based on questionnaires to be answered.
SRS-2 (Interpersonal Responsiveness Scale)
(mark-sheet method)
-PHQ-4 (4-item scale for depression and anxiety)
-GSRS (Gastrointestinal Symptom Checklist)
-BS Score (record of changes in stool status)
(2) Tests performed at medical institutions
Gazefinder, a line-of-sight measurement device
THIS INSTRUMENT IS ONE TOOL TO OBJECTIVELY EVALUATE ASD CHARACTERISTICS BASED ON HOW YOU LOOK AT THINGS, SUCH AS WHERE YOU LOOK AT A 2-MINUTE VIDEO. THE TEST IS LESS BURDENSOME FOR THE CHILD AS IT CAN BE PERFORMED BY SIMPLY LOOKING AT THE SCREEN.

(3) Stool samples collected at home and mailed to us for testing
-16S rRNA gene analysis (fecal gene test)
– This is a bacterial gene test to examine the distribution of bacteria in feces.
– Stool for storage
(4) Online Diaries
Please enter your daily activities and changes in behavior in the online diary, although this does not apply to the inspection. (You can use your computer or smartphone to input the information.)
・Please input your waking and sleeping times, food records, defecation records, moods, etc.
Please enter any changes in behavior, such as tantrums or obsessions, in the free-entry field.
(5) Schedule
The following is a summary of the timing of the various test items.
If you have any questions, please contact the Clinical Research Office.
Table 3. schedule table
-883x1024.jpg)
(6) Provision of medical care after completion of research
AFTER TREATMENT IN THIS STUDY IS COMPLETED, FMT CAN STILL BE PERFORMED AS NEEDED. IN THAT CASE, THIS METHOD WILL BE FREE OF CHARGE UNTIL SUCH TIME AS IT IS COVERED BY INSURANCE IN THE FUTURE.
Planned implementation period and target number of cases
1) Recruitment period for study subjects
from the date of jRCT release until the target number of cases (30) is reached.
2) Observation period for clinical studies
from the first FMT until 30 weeks.
6. anticipated burdens
(1) Transportation and time consumption
Since the frequency of visits to the clinic will increase compared to the regular clinic, there is a possibility that the time and transportation costs will increase. (The time and transportation costs for the free medical examination before the start of the clinical research, 6 visits for transplantation, and 3 visits for observation will also increase). Pre-transplant and 8 weeks post-transplant. The 16S rRNA gene test is performed prior to transplant and 8 and 30 weeks after the start of transplantation. This test requires you to collect your child’s stool samples at home with a special kit and send them by mail, but it takes time and effort to collect and send the stool samples.
(2) Details and costs of self-funded medical treatment prior to the start of clinical research
Costs incurred prior to inclusion in clinical research(excluding tax)
| Examination fees (clinical findings (examination), height, weight, blood pressure, temperature, auscultation, pulse, SpO2) | About 10,000 yen/time *Varies depending on time |
*Various other tests (urine test, blood test, etc.) may be added as necessary.
*There are no fees for the six transplantation consultations and transplantation procedures and three post-observation consultations.
(3) Medication during participation in clinical research
Depending on the drug, you may be asked to discontinue taking the drug while participating in the study.
(4) Adverse events
FMT IS EXPECTED TO BE A METHOD WITH FEWER ADVERSE EVENTS. ADVERSE EVENTS OF FATIGUE AND CONSTIPATION WERE OBSERVED IN ONE CHILD OUT OF 14, DUE TO THE ADMINISTRATION OF THE INTRAVENOUS SOLUTION, BUT THEY WERE MINOR AND TEMPORARY. HOWEVER, THE POSSIBILITY OF AN INCREASE IN ADVERSE EVENTS CANNOT BE RULED OUT IF THE NUMBER OF CASES INCREASES.
IN THE ADMINISTRATION OF SHIN-1-CONTAINING INTRAVENOUS SOLUTIONS FOR DISEASES OTHER THAN ASD (ABOUT 50 CASES) CONDUCTED UNDER FREE TREATMENT SINCE APRIL 2022, ADVERSE EVENTS SUCH AS ABDOMINAL PAIN, CONSTIPATION, DIARRHEA, FEVER, ETC. WERE OBSERVED IN SOME CASES, BUT ALL EVENTS WERE MILD AND TEMPORARY.
We will make every effort to ensure the safety of your child during the clinical research and subsequent consultations, and in the event of a serious adverse event (all unfavorable events, including side effects, regardless of causal relationship to the intravenous administration), we will do our best to ensure the safety of your child by taking prompt and appropriate action to ensure safety as our first priority.
Side effects vary from person to person and do not always occur. Any medication can cause side effects, so if you experience any unusual symptoms, please contact your doctor or the contact information or consultation service at the end of this document.
7. participation in this clinical research
Your child and you are free to decide whether or not to participate in this study. You may decline to participate. Whatever you decide, it will not be to the detriment of your child or you. Please read the instructions carefully and contact the Clinical Research Office if you are interested in participating.
After careful consideration, including consultation with your family, please visit the medical institutions (6 facilities) where the principal investigator is enrolled and make a decision at your child’s and your own free will.
If you are willing to cooperate, please sign the consent form.
8. withdrawal of consent
You may withdraw your consent at any time after agreeing to participate in this study, and even if you decline, you will not be disadvantaged in your future treatment. If you wish to withdraw your consent, please do not hesitate to inform your physician.
9. method of disclosing information on research
An outline of the purpose and methods of this study will be registered with the database (jRCT: Japan Registry of Clinical Trials, URL : https://jrct.niph.go.jp/ ) maintained by the Ministry of Health, Labour and Welfare prior to the implementation of the study, and will be made publicly available.
You can also view the progress and results of the research. Please note that personal information is withheld from the public so that individuals cannot be identified.
10. expenses
(1) THE INTRAVENOUS SOLUTION WILL BE PROVIDED FREE OF CHARGE BY SYMBIOSIS, INC. THEREFORE, THERE IS NO ADDITIONAL FINANCIAL BURDEN ON THE SURROGATE OF THE CHILD UNDERGOING FMT.
(2) When a patient undergoes a medical examination before participation in this study is confirmed, the patient may be charged 10,000 to 20,000 yen as out-of-pocket expenses. (Medical examination fee, etc.)
(3) No honorarium or other payment will be made to you.
(4) Please note that you will be responsible for your own transportation and lodging costs if you come from a distant location.
11. compensation in case of health damage
Although this research will be conducted with the utmost care, in the unlikely event that a child suffers health problems related to this research, we will provide the best possible treatment as soon as possible. Compensation for any damage to health will be provided in accordance with the terms and conditions of the liability insurance policy (clinical research insurance).
Name of the medical institution and the name, title, and contact information of the physician in charge
Principal Investigator] (the researcher who oversees the entire study)
Tanaka Clinic, Jinzenkai Medical Corporation, President, Zen Tanaka
| Medical Corporation Nizenkai Tanaka Clinic | 1F Denki Kan Building, 2-3-8 Ikunonishi, Ikuno-ku, Osaka, Japan 06-6711-3770 06-6711-3770 | Yoshinori Tanaka | board chairman | https://www.tanaka-cl.com/fmt.html |
| Luke’s Ashiya Clinic | 8-2 Oharacho, Ashiya-shi, Hyogo, Japan 0797-23-6033 0797-23-6033 | Masahiko Shirotani | director | https://www.lukesashiya.com/feature/flora.html |
| Medical Corporation Kiwakai Kitamura Clinic | 4-3-8 Nishiki-cho, Onojo, Fukuoka, Japan 092-581-6640 092-581-6640 | Kunihiro Kitamura | director | http://www.kitamura.or.jp/contents_fmt.html |
| Medical Corporation Yuakai Cute Internal Medicine Clinic | 3-7-14 Higashinakahama, Joto-ku, Osaka City, Osaka, Japan 06-6962-3133 06-6962-3133 | Yuichi Kawai | board chairman | https://www.kawai-medical.com/diagnosis02/ |
| Futamatakai Medical Corporation Natural Art Clinic | Android Building 2F, 6-5 Rokubancho, Chiyoda-ku, Tokyo 03-6256-8448 03-6256-8448 | Yasuhito Mikawa | director | https://naturalartclinic.com/alternative-therapy/1549 |
| Dr. Reiko Haruna, Deputy Director of theHaruna Clinic ! | 1-3-13-202 Nishimikuni, Yodogawa-ku, Osaka-shi, Osaka 06-4807-5130 06-4807-5130 | Reiko Haruna | vice president (of a hospital, etc.) | https://www.haruna-clinic.com/541-2 |
13. check with the Clinical Research Submission and Release System
The information is available on the jRCT (Japan Research Council’s system for the submission and release of clinical research data).














